THE MCKAY LABORATORY


Brian S. McKay, PhD
Professor
Department of Ophthalmology
and Vision Science
Brian McKay was born and educated in Wisconsin. Dr. McKay obtained his BS in Biology from the University of Wisconsin–Milwaukee and his PhD in cell biology and anatomy from the Medical College of Wisconsin in 1995. Postdoctoral training in protein chemistry was obtained at the Scripps Research Institute in La Jolla, California. Dr. McKay was recruited to the Department of Ophthalmology at Duke University in 1997 where the independent McKay Laboratory began with studies of RPE pigmentation, the fundamental question in the laboratory was initiated. In 2002, Dr. McKay was recruited to join the Department of Ophthalmology and Vision Science at the University of Arizona to study Age-related Macular Degeneration (AMD) and glaucoma, the leading causes of irreversible blindness. Dr. McKay remains at University of Arizona as a research scientist, principal investigator of the McKay Lab, and as a teacher, facilitating classes for medical students, and training students from high school to graduate students in laboratory research methods and the concepts behind experimental design.
THE McKAY LABORATORY TEAM
Brian S. McKay, PhD, Principal Investigator
BSMcKay@eyes.arizona.edu
Nicole Congrove, Senior Laboratory Technologist
NCongrove@email.arizona.edu
|
The McKay Lab |
PIGMENTATION AND VISION
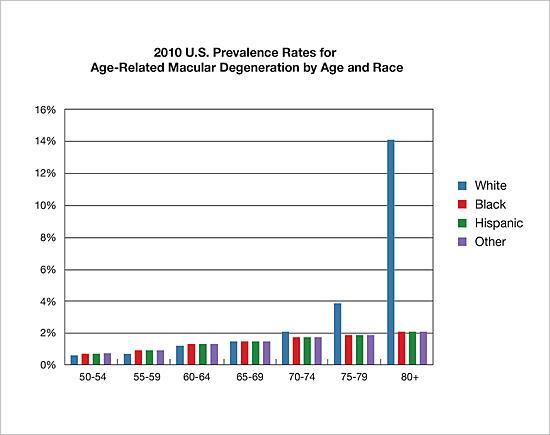
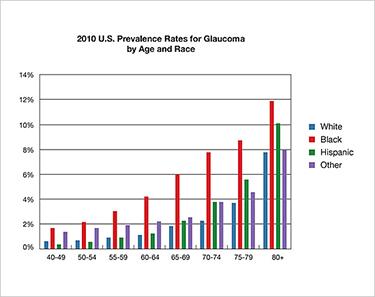
The two most common causes of irreversible blindness are Age-related Macular Degeneration (AMD) and glaucoma. The incidence or risk of both diseases is linked to race and pigmentation, but in opposite ways. Those with darker pigmentation appear protected from AMD, but are at significantly greater risk of glaucoma.
|
|
|
Age-related Macular Degeneration (AMD)
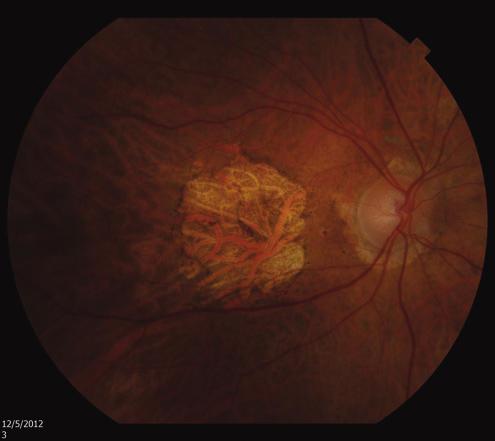
McKay BS, Schwartz SG. Pigmentation and Macular Degeneration:
Is There a Role for GPR143? J Ocul Pharmacol Ther. 32: 3–4.
doi:10.1089/jop.2016.29007.bsm
The McKay Lab specifically investigated one of the pigmentation genes what was a potential orphan G-protein coupled receptor (GPCR). We discovered that the ligand for this receptor (GPR143) is L-DOPA [1], and that the receptor controls RPE expression of the most potent neurotrophic factor in the eye (PEDF) [1,2] which sustains retinal neuron survival. Most recently we investigated whether those taking L-DOPA for movement disorders, such as Parkinson’s Disease were protected from AMD. Using the medical records of approximately one-fourth of the Unites States, over 87,000,000 people, we discovered that L-DOPA appeared protective from AMD both reducing the risk of ever developing the disease, and delaying the onset for those who do develop the disease by 8 years [3,4].
Based on these results we have partnered with several Tucson Clinicians to initiate a prospective clinical trial to determine whether L-DOPA stops, prevents, and/or treat AMD. These FDA approved trials can be found at clinicaltrials.gov # NCT03022318 and NCT03023059.
Glaucoma
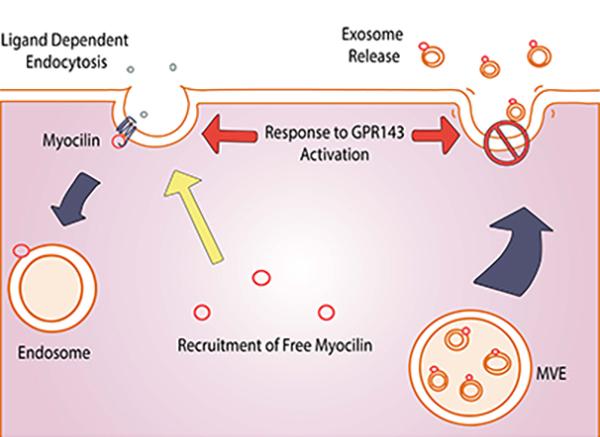
Locke CJ, Congrove NR, Dismuke WM, Bowen TJ, Stamer WD, McKay BS.
Controlled exosome release from the retinal pigment epithelium in situ.
Exp Eye Res.
The first gene linked to genetic primary open angle glaucoma, POAG, the most common form of the disease, was myocilin. The myocilin gene and protein are expressed throughout the body, but mutations only cause glaucoma suggesting some ocular specific dysfunction when the protein is mutated. Interestingly, myocilin is both a cytoplasmic protein and an extracellular protein which has made assigning a function for the protein difficult. We discovered that myocilin is not a traditionally secreted protein, but rather leaves cells on the surface of exosomes [5–7]. After further study we determine that myocilin associated with membranes as part of a large protein complex [8] with properties similar to those of the SNARE family that function in vesicle trafficking. We subsequently found that myocilin is recruited to the early endosome during receptor mediated endocytosis of a GPCR. The GPCR that recruits myocilin to the endosome is the L-DOPA receptor from the pigmentation pathway (GPR143) [9]. Further reinforcing the linkage between myocilin and the pigmentation GPCR for L-DOPA, we discovered that L-DOPA immediately halts myocilin exosome release from RPE [7]. Exosomes are small extracellular vesicles that function in extracellular communication. Our results suggest that mutations in myocilin may disrupt this communication pathway, and further that the L-DOPA receptor (GPR143) may influence myocilin communication and underlie the racial bias of POAG.
Selected Publications Referenced Above
1. Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008;6: e236. doi:10.1371/journal.pbio.0060236
2. Falk T, Congrove NR, Zhang S, McCourt AD, Sherman SJ, McKay BS. PEDF and VEGF-A output from human retinal pigment epithelial cells grown on novel microcarriers. J Biomed Biotechnol. 2012/05/02. 2012;2012: 278932. doi:10.1155/2012/278932
3. Brilliant MH, Vaziri K, Connor TB, Schwartz SG, Carroll JJ, McCarty CA, et al. Mining Retrospective Data for Virtual Prospective Drug Repurposing: L-DOPA and Age-related Macular Degeneration. Am J Med. Elsevier; 2016;129: 292–8. doi:10.1016/j.amjmed.2015.10.015
4. McKay BS, Schwartz SG. Pigmentation and Macular Degeneration: Is There a Role for GPR143? J Ocul Pharmacol Ther. 32: 3–4. doi:10.1089/jop.2016.29007.bsm
5. Hardy KM, Hoffman EA, Gonzalez P, McKay BS, Stamer WD. Extracellular trafficking of myocilin in human trabecular meshwork cells. J Biol Chem. 2005/06/10. 2005;280: 28917–28926. doi:10.1074/jbc.M504803200
6. Perkumas KM, Hoffman EA, McKay BS, Allingham RR, Stamer WD. Myocilin-associated exosomes in human ocular samples. Exp Eye Res. 2006/11/11. 2007;84: 209–212. doi:10.1016/j.exer.2006.09.020
7. Locke CJ, Congrove NR, Dismuke WM, Bowen TJ, Stamer WD, McKay BS. Controlled exosome release from the retinal pigment epithelium in situ. Exp Eye Res. Elsevier Ltd; 2014;129: 1–4. doi:http://dx.doi.org/10.1016/j.exer.2014.10.010
8. Dismuke WM, McKay BS, Stamer WD. Myocilin, a component of a membrane-associated protein complex driven by a homologous Q-SNARE domain. Biochemistry. 2012/04/03. 2012;51: 3606–3613. doi:10.1021/bi300073r
9. McKay BS, Congrove NR, Johnson A a., Dismuke WM, Bowen TJ, Stamer WD. A role for myocilin in receptor-mediated endocytosis. PLoS One. 2013/12/25. 2013;8: e82301. doi:10.1371/journal.pone.0082301
Selected Publications
Peer-reviewed
- Pigmentation and vision: Is GPR143 in control? McKay BS. (2018). Journal of Neuroscience Research
- Mining Retrospective Data for Virtual Prospective Drug Repurposing: L-DOPA and Age-related Macular Degeneration. Brilliant MH, Vaziri K, Connor TB Jr, Schwartz SG, Carroll JJ, McCarty CA, Schrodi SJ, Hebbring SJ, Kishor KS, Flynn HW Jr, Moshfeghi AA, Moshfeghi DM, Fini ME, McKay BS. (2016). American Journal of Medicine
- Controlled exosome release from the retinal pigment epithelium in situ. Locke CJ, Congrove, NR, Dismuke MW, Bowen, TJ, Stamer DW, Mckay BS. (2014). Experimental Eye Research
- A Role for Myocilin in Receptor-Mediated Endocytosis. McKay BS, Congrove NR, Johnson AA, Dismuke MW, Bowen, TJ, Stamer DW. (2013) PLOS One
- PEDF and VEGF-A Output from Human Retinal Pigment Epithelial Cells Grown on Novel Microcarriers. Falk T, Congrove NR, Zhang S, McCourt AD, Sherman SJ, McKay BS. (2012). J. Biomed. Biotechnol.
- Myocilin, a component of a membrane-associated protein complex driven by a homologous Q-SNARE domain. Dismuke WM, McKay BS, Stamer WD. (2012). Biochemistry
- L-DOPA Is an Endogenous Ligand for OA1. Lopez VM, Decatur CL, Stamer WD, Lynch RM, Mckay BS. (2008). PLOS Biology
If you would like to help support macular degeneration research at the McKay Lab, please make a donation. GIVE TODAY!
MACULAR DEGENERATION RESEARCH FUND